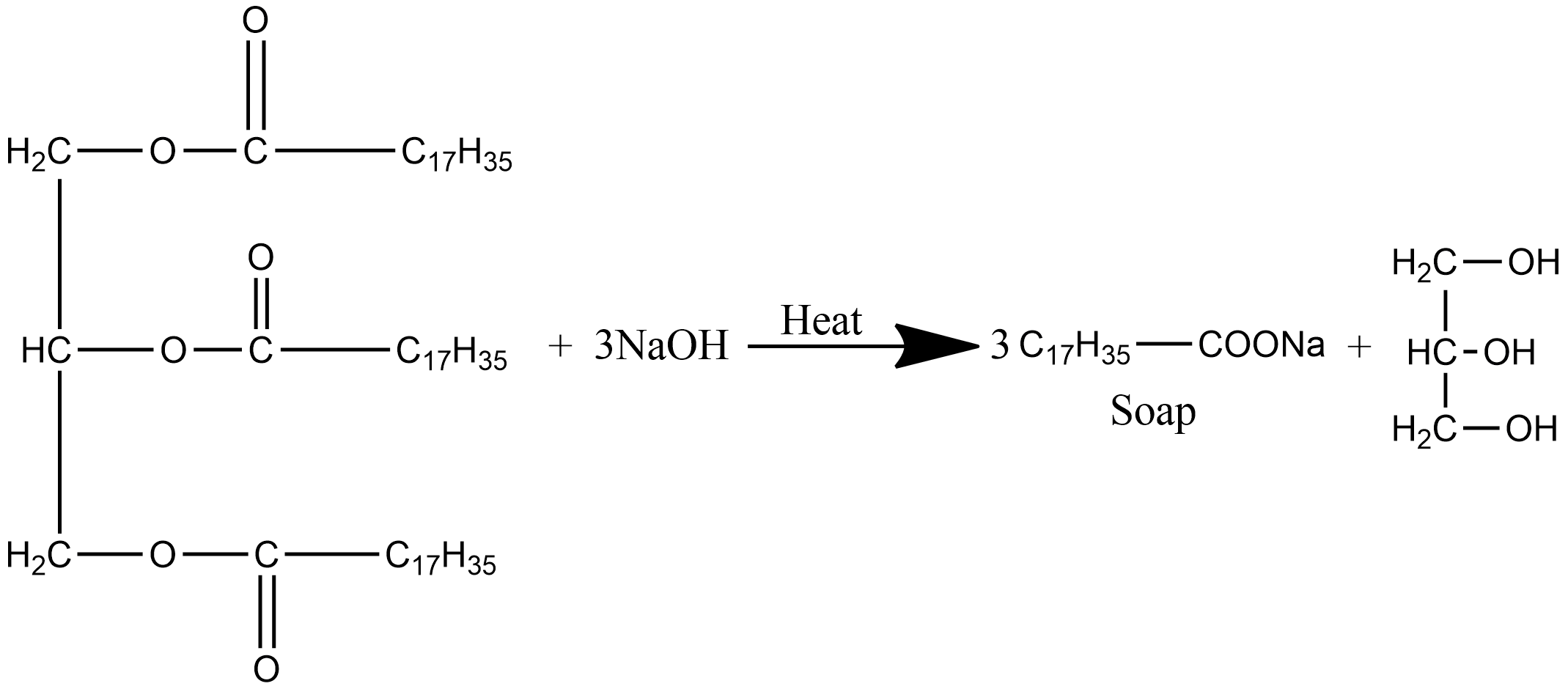
Complete and balance the following chemical equation for the production of soap. Does this reaction produce a solid or liquid soap? Justify your answer. | Homework.Study.com
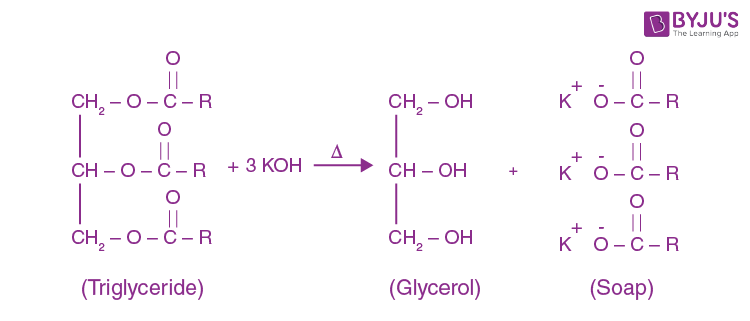
Saponification - Definition,Reactions, Mechanism, Examples, Saponification Value, Uses, FAQs with Videos on Saponification.



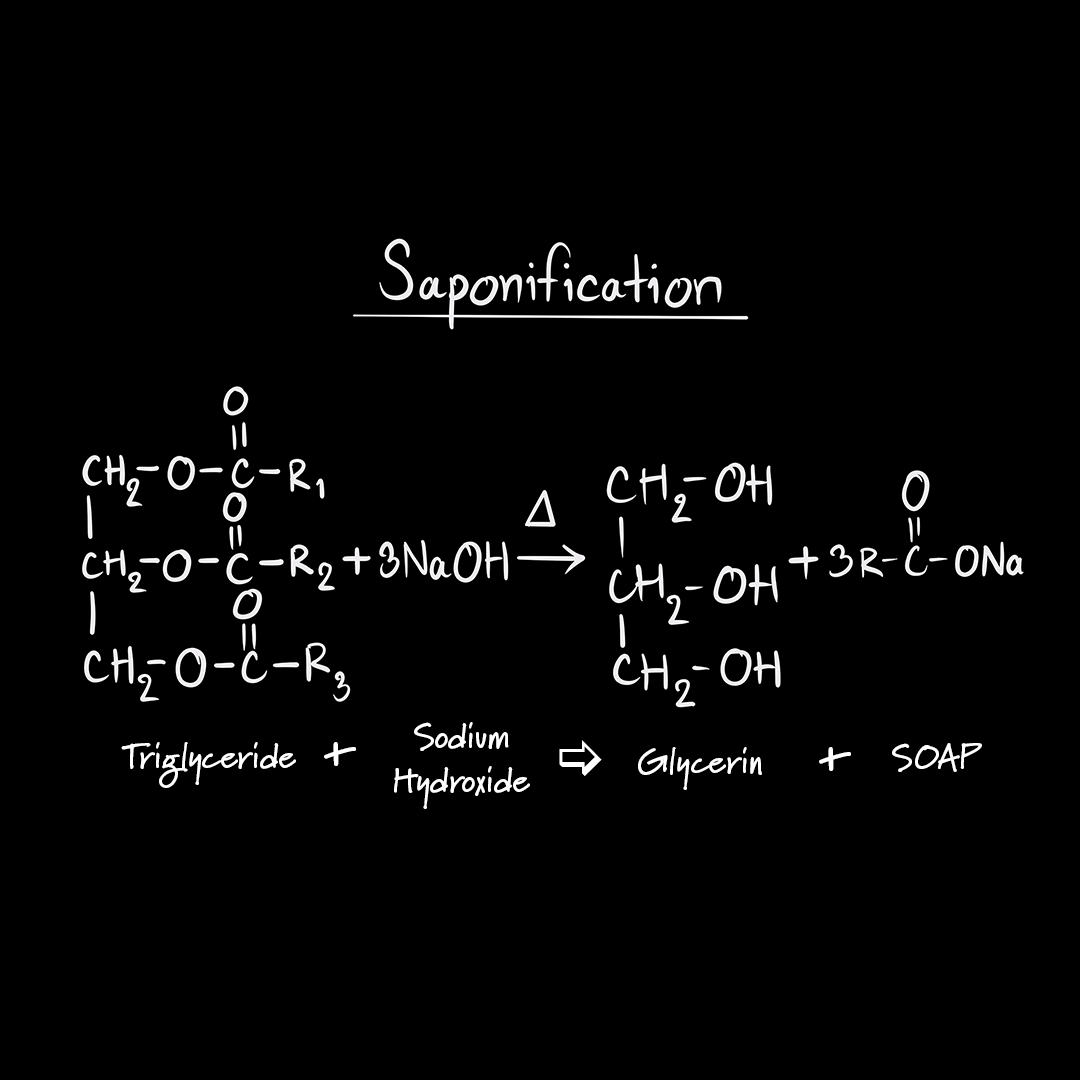
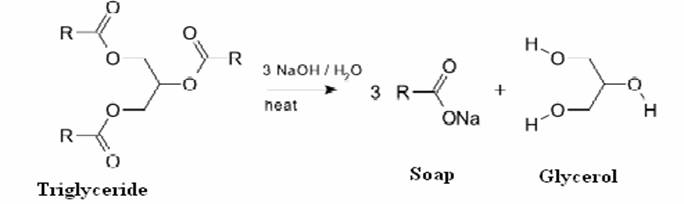
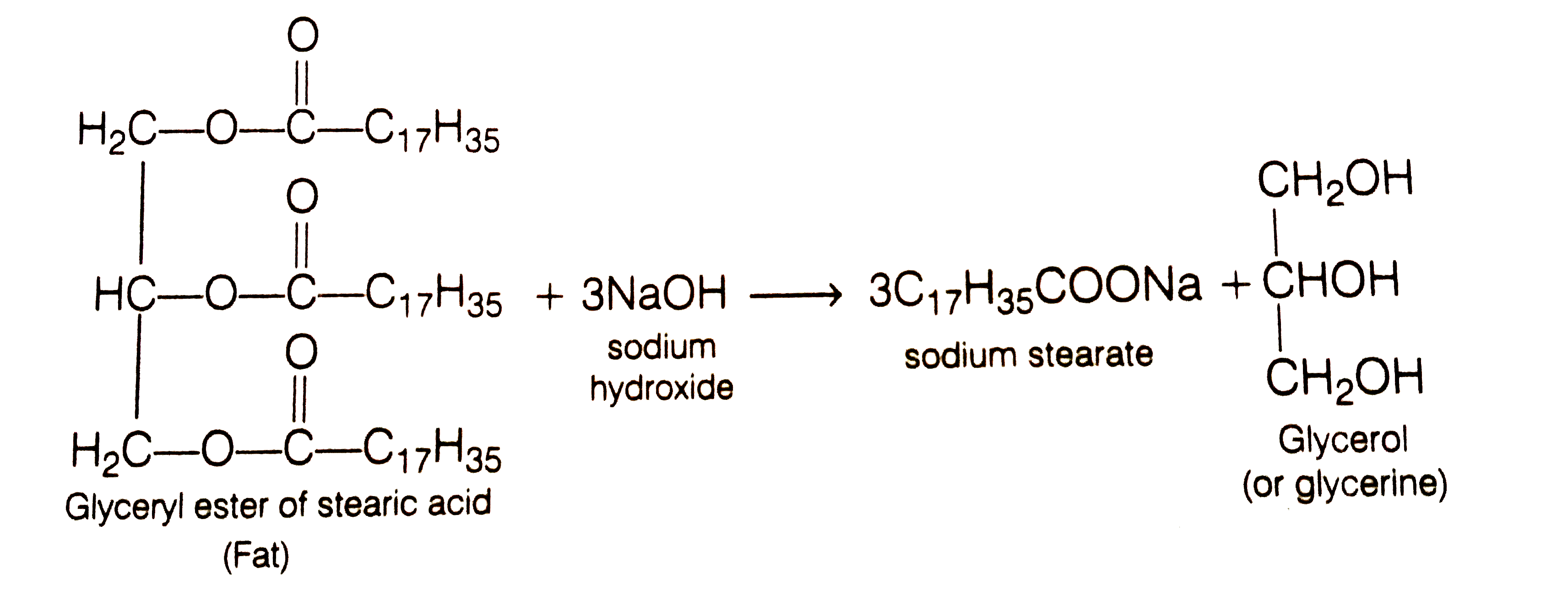
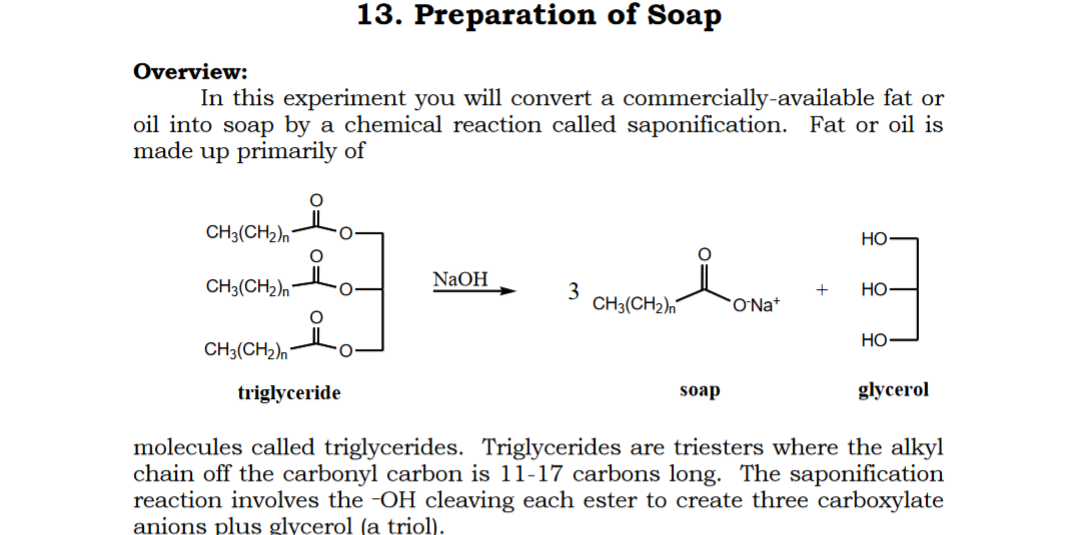

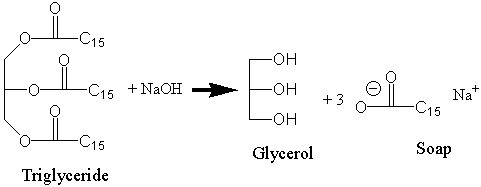
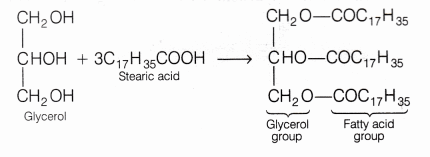
![Saponification Reaction for Soap Product [14]. | Download Scientific Diagram Saponification Reaction for Soap Product [14]. | Download Scientific Diagram](https://www.researchgate.net/publication/332053508/figure/fig1/AS:741429492068359@1553781974829/Saponification-Reaction-for-Soap-Product-14.png)
:max_bytes(150000):strip_icc()/close-up-of-soap-on-white-background-912188980-5b631a00c9e77c00256c6340.jpg)


:max_bytes(150000):strip_icc()/Saponification-56a132ca5f9b58b7d0bcf749.png)