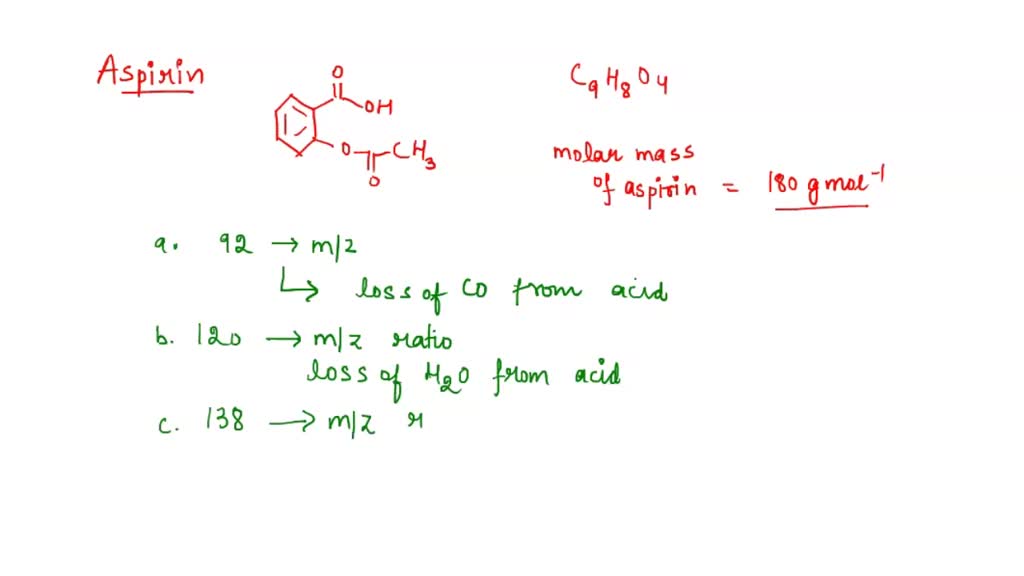
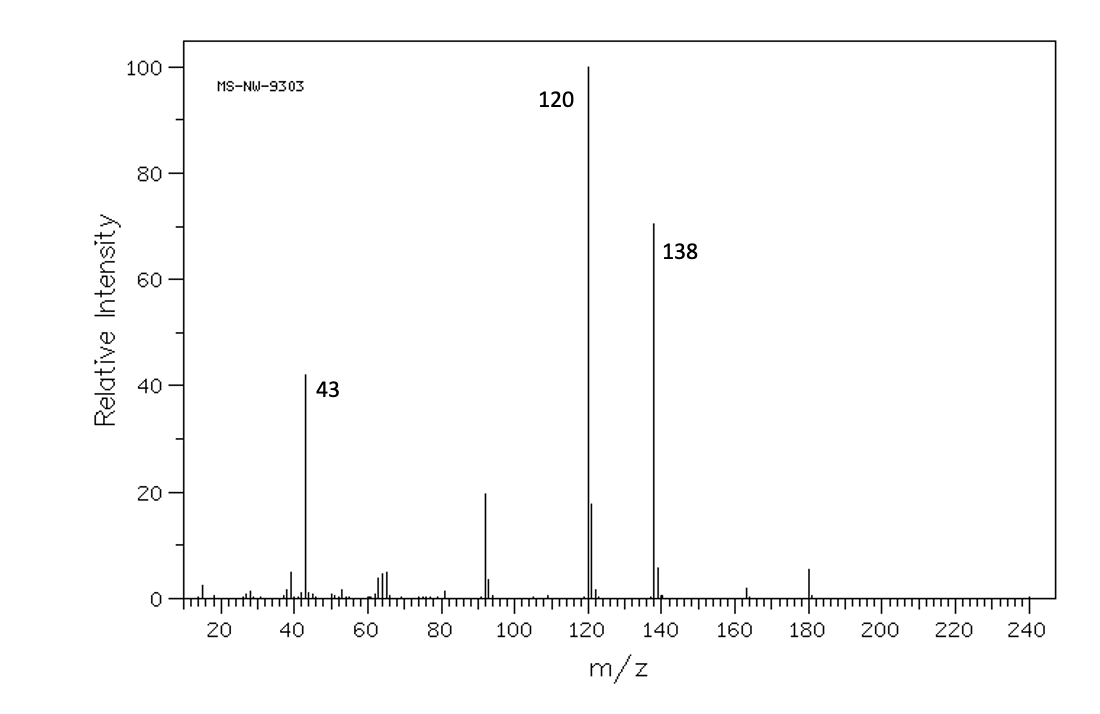
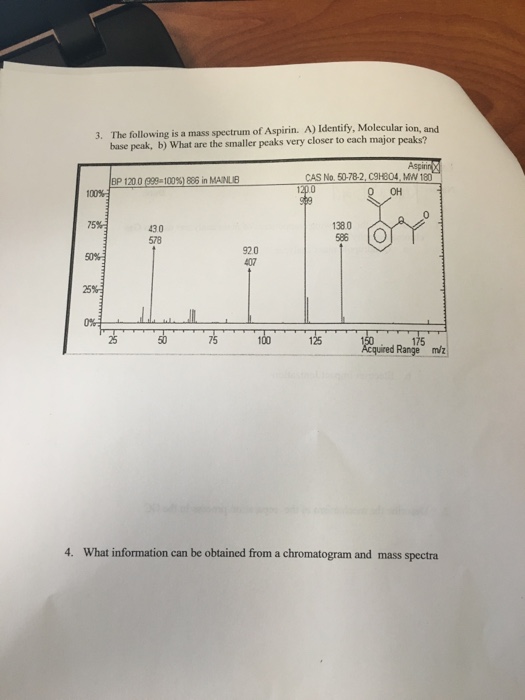
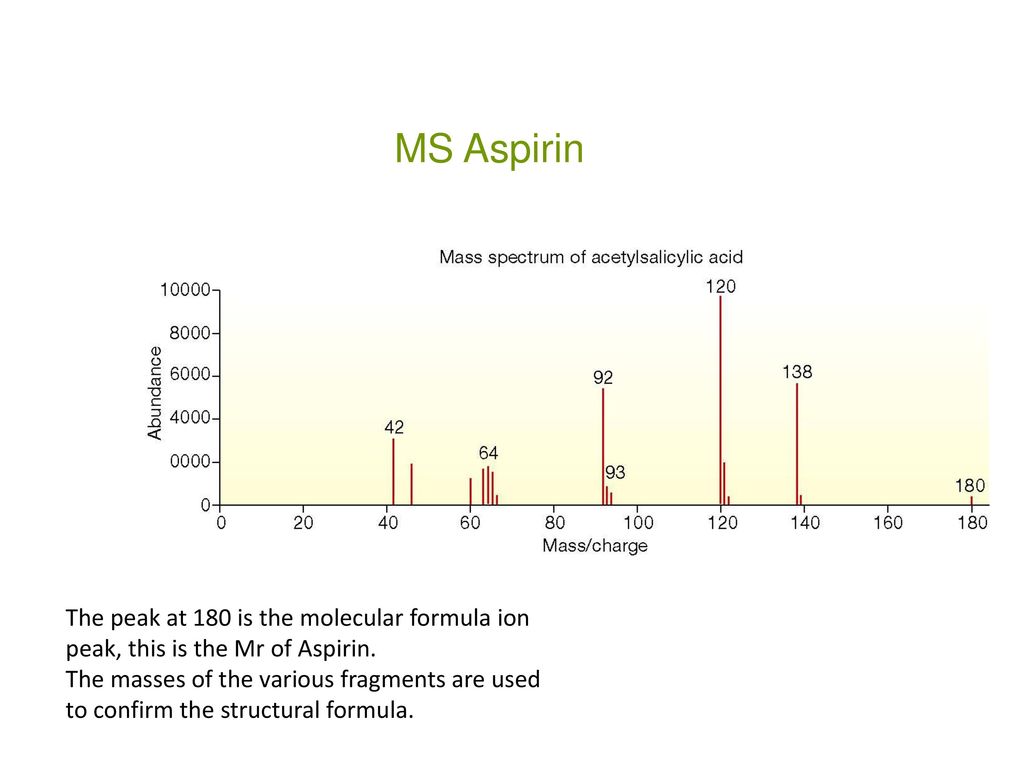
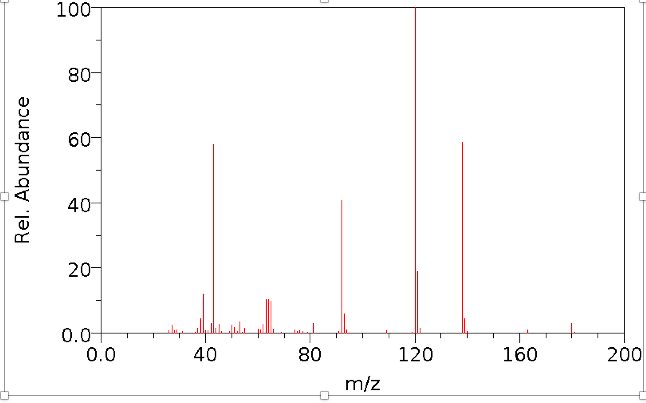
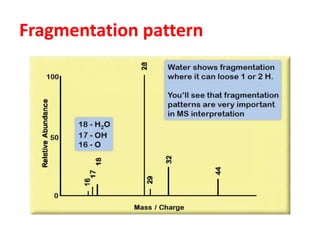
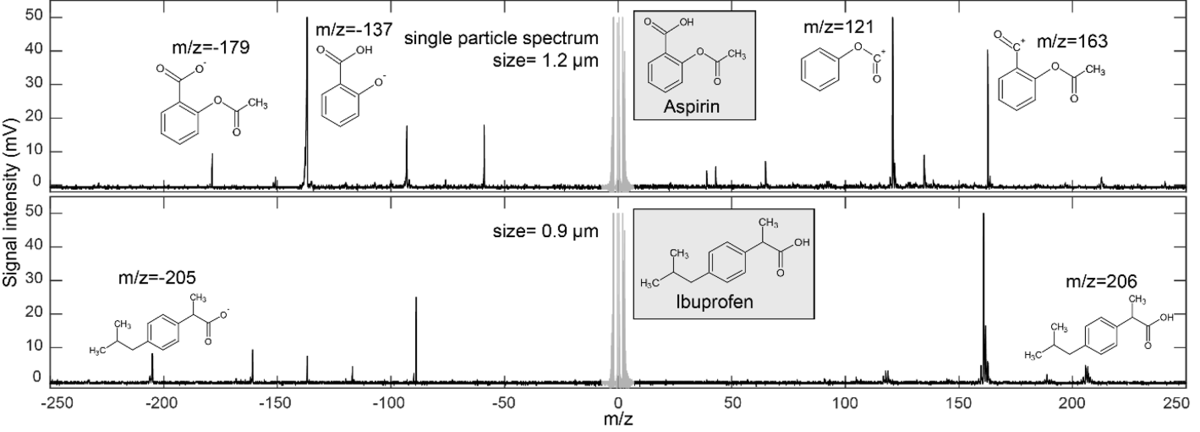
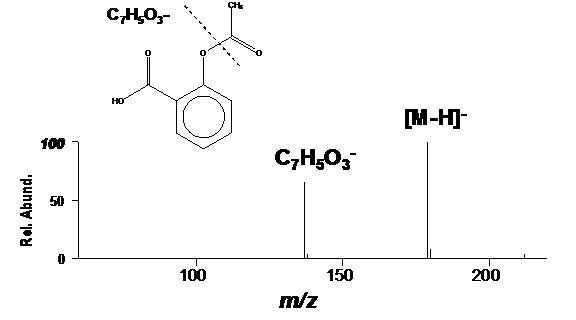Figure 1 from Isolation, Characterization of a Potential Degradation Product of Aspirin and an HPLC Method for Quantitative Estimation of Its Impurities. | Semantic Scholar

Proposed CID fragmentation mechanisms for the major fragment ions from... | Download Scientific Diagram

Collision‐induced fragmentation pathways including odd‐electron ion formation from desorption electrospray ionisation generated protonated and deprotonated drugs derived from tandem accurate mass spectrometry - Williams - 2006 - Journal of Mass ...

Aspirin revealed: A cationization strategy for detecting acetylsalicylic acid by MALDI mass spectrometry - ScienceDirect

Substructures obtained by fragmentation of Aspirin, the "A" represents... | Download Scientific Diagram
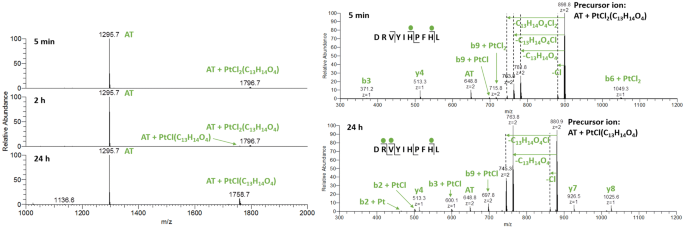
Top-down mass spectrometry reveals multiple interactions of an acetylsalicylic acid bearing Zeise's salt derivative with peptides | JBIC Journal of Biological Inorganic Chemistry